Evidence expectations are high
When evidence is incomplete, reviews slow teams down. Preserve movement history and change context by default.

Maintain defensible evidence and controlled workflows for regulated operations. Standardize approvals, adjustments, and movement history so teams can respond quickly to reviews and inspections.
Operate with controlled workflows and defensible evidence: preserve approvals, traceability, and export-ready reporting so regulated reviews don’t disrupt operations.



Biotech and pharma operations face regulated workflows and high standards for evidence. Inventory operations connect movements, controls, and reporting—so teams stay compliant while moving quickly across sites.

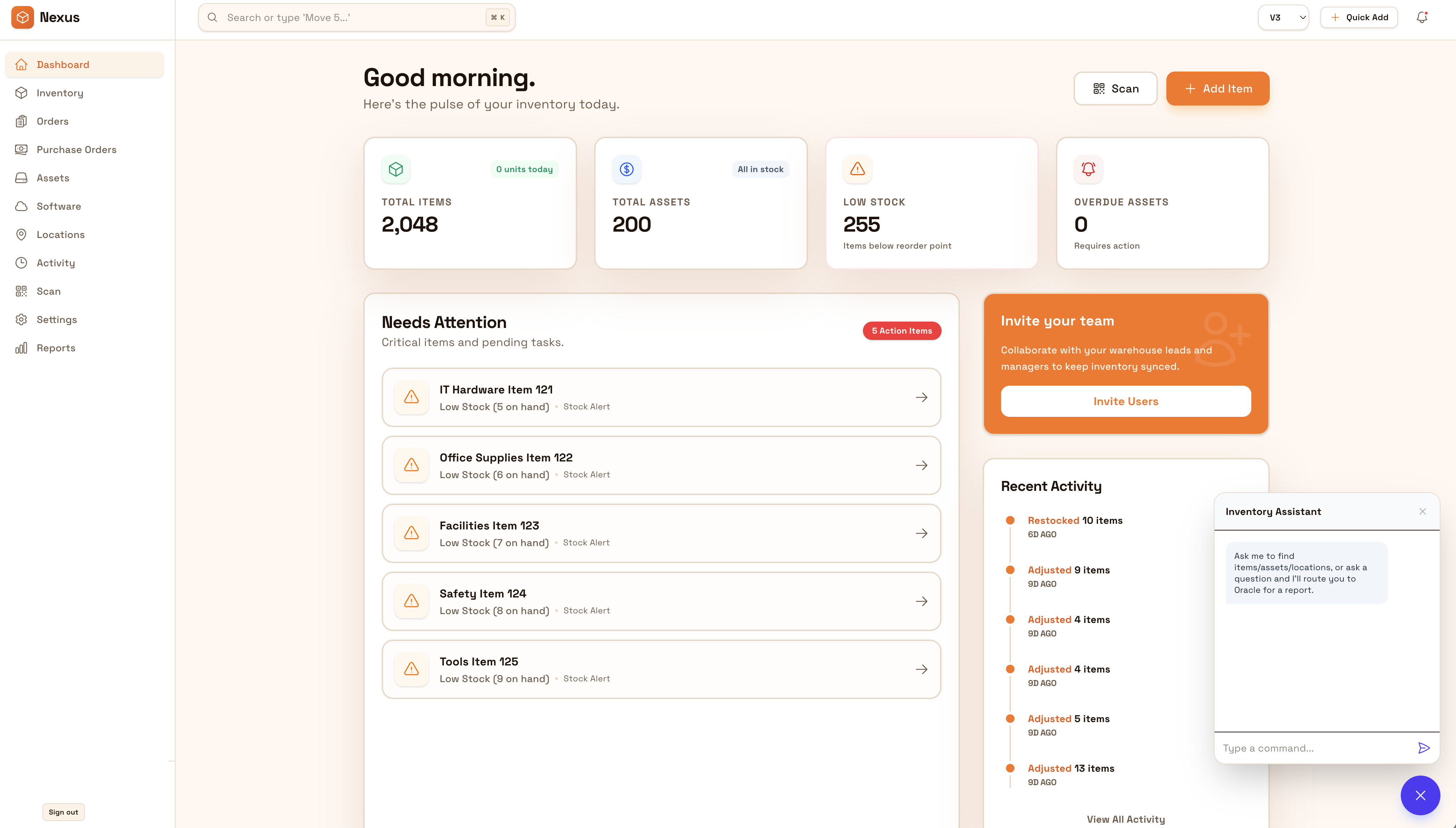
Most teams start here. IO helps you reduce drift, keep accuracy high, and maintain a trail you can trust.
When evidence is incomplete, reviews slow teams down. Preserve movement history and change context by default.

Adjustments without approvals create risk. Use controlled workflows so changes are governed and explainable.

Sites that operate differently produce inconsistent reporting. Standardize core workflows while keeping flexibility where it matters.

IO standardizes tracking, controls, and evidence—so teams can operate confidently across sites and stakeholders.

• Inventory and assets by controlled locations • Transfers with traceable destinations • Adjustments with approvals and reasons • Audit trail for movements and lifecycle events • Exportable evidence for reviews and inspections
Define sites and sub-locations so movement is always traceable.
Require approvals and reasons for adjustments and disposals.
Run repeatable counts and reconcile variance with documented outcomes.
Export movement history and summaries to respond quickly to inspections and reviews.
Tell us your compliance and operational requirements. We’ll recommend the right rollout.
Use roles, approvals, and audit history to make changes governed and defensible. Evidence should be ready before it’s requested.

The biggest wins are usually accuracy, speed, and defensible accountability.

Yes—use approvals for sensitive adjustments and controlled changes.
Yes—movement history and exports provide defensible reporting for reviews.
Yes—location structure and workflows can be standardized across facilities.
Yes—documented reasons and clean history shorten investigations.
Start with a pilot workflow and a controlled location map, then expand.